Take a look at your cell phone, PDA or laptop: due to the rapid technological advances have become widely available devices, which have ten or fifteen years can be seen only in science fiction films. And among this riotous color blooming high-tech industry is a perfect anachronism conventional batteries. Just think, the principles that underlie all modern batteries were opened even in the last century, but much earlier. And since they have undergone is not so great changes, which mainly consisted of reducing the size and use of improved materials. It would seem that the world GHz and nanotechnology is the "old" should take a back seat. But with the advent of many modern mobile devices (players, PDAs, cameras and camcorders, laptops, etc) we are witnessing the opposite tendency - the batteries and the batteries are not less important part than the processors. It all depends precisely on the capacity of the power source. And without it, even the most feature-rich gadgets will be absolutely useless. In fact, such a solid age of technology - it's a good thing. Over this period, scientists and researchers have studied the problem in detail. Modern "duraselly" far removed from the battery Volts two centuries ago. And now, manufacturers are spending big money to improve the parameters of their products and reduce their size. A motor of this process is a constant striving toward miniaturization of electronics manufacturers. All the latest developments in this area are trying to meet the needs of modern mobile technology. The fact that they even work in new ways, is not so, as radios or flashlights. All of these digital cameras, PDAs, and CD-MD-MMC-MP3-players need batteries that can withstand sudden surges that occur during power screens, unwinding discs, and out of hibernation. In contrast, computer companies, holy chtyaschih Moore's law, the firms producing the batteries, have no illusions about the nearest (and even not so) future. Previous decades (!) Taught them not to wait for the miraculous emergence of new technologies that will increase the battery capacity in half. On the contrary, we must work hard, gradually improving available. Suffice it to say that over ten years of lithium-polymer battery life of this technology has not been fully exhausted, and therefore the best minds of the industry continues on the percentage by half a percentage point increase in their specific capacity. Batteries have come a long way of development, but they still are not enough to serve people. Next, we'll tell you about the history of batteries, as well as try to understand what lies ahead. Well, to begin with a look at how they work and what they have inside. Batteries - are devices that store energy, which they then give that same energy-consuming device. However, under this definition includes as flywheels or, say, the clock spring. Unfortunately, at this point in the Russian market clockwork models of cellular phones or PDAs are not represented at all, so we leave this interesting topic to better times. Omit also the story about the lead-acid batteries - despite the fact that they have a huge capacity, mobility (not to be confused with the car) leaves much to be desired. What we usually mean by the word "battery" can be described as follows: an isolated system in which chemical processes occur, resulting in the generated electric power. The emergence of portable computers, as well as many other mobile "stuff", gave a new impetus to the development of autonomous power supply technologies. If you look at conventional computers, they are fed from the network, and therefore practically do not use batteries. As an exception can be called a CMOS-battery on the motherboard, batteries, uninterruptible power supply (UPS'ov), and, on the little things, "fingers" that are inserted into various wireless mice, keyboards, etc. How different mobile devices: there is not even argue about anything, it is difficult to name even one, which would not have stood a battery (or battery). In addition, when all the variety of shapes and sizes, by and large, they all use virtually the same batteries. That is to say, and a mobile phone and a laptop equipped with the same Li-Ion-batteries, although the shape and the capacity to compare them is difficult.At the same time, the concept of all batteries manufactured for mass consumption is virtually the same. Two electrodes - a cathode and anode - are made of two different metals (strictly speaking, they should have different degrees of oxidation). The space between them completed by a third material called an electrolyte. Wide choice of components allows you to create a unified scheme are many types of batteries that are sometimes diametrically opposed properties, different specific capacity (ratio of maximum battery to its volume) and the nominal voltage.
History
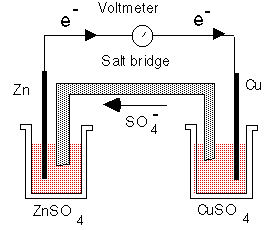
It is considered that the basic principles of which are used to this day, were discovered in the late XVIII century Italian physicist and naturalist, Alessandro Volta (1745-1827). It was then working at the University of Pavia, he became interested in "animal electricity", open a few years earlier by his compatriot Luigi Galvani (in his honor electrochemical batteries are often called galvanic). Volta proved that it is the current produced by the contact of two different metals, causing a decline in muscle in frog legs. He therefore rejected the idea Galvani that electricity is produced in muscles. To prove his point, he filled two cups with brine and combined them with metal edges. One end of these arcs were copper and one zinc. They were established so that each bowl was one electrode of each type. This construction and the first battery produces electricity by chemical reaction between two metals in solution. In 1800 he perfected it, creating his famous "Voltaic pile", the first DC power source. He represented the 20 pairs of dots, made of two different metals, laid pieces of leather or cloth moistened with a saline solution. In recognition of Italian scientist, his name has been named one of the voltage - volts.
 |
Other experimenters have noticed the results. They have improved the Voltaic pile, creating new types of batteries. For example, in 1836 the English chemist John Daniell put copper and zinc electrodes in a container of sulfuric acid. This battery is called "planar element" or "element of Daniel." Three years later, another Englishman, William R. Grove, Oxidizer added to prevent hydrogen accumulation near the cathode, which reduces the voltage at the output. There were other attempts to improve the original design, but none of these primitive devices are not used nowadays. The first significant breakthrough was made Frenchman Gaston Plante. In 1859 he spent an interesting experience, outwardly similar to that done Volta. In his galvanic cells were used as electrodes for lead plates and electrolyte is dilute sulfuric acid. Plante connected to elements of the DC, and some time to charge the battery. The instrument itself was generating electricity, producing almost all the energy spent on exercises. And it can be recharged many times. That is exactly what appeared the same lead-acid battery that it will still be used in all cars produced. Another device is a long-liver has been developed and patented by another French inventor, Georges Leklanshe in 1866. Named in honor of his element served as the prototype of the modern "dry" batteries, although initially it was such a name did not match. The fact that in the version proposed Leklanshe, the electrolyte was a liquid. At the same now manufactured batteries has been replaced by jelly to prevent leakage of the contents and damage to equipment that feeds the battery. In the rest, during which time technology has changed little. As a half-century ago, Dry elements are zinc cup (anode), which is inserted into a graphite rod (cathode) and the inner space is filled with electrolyte. Using this technology produce the cheapest and massive power supplies, which are inserted into a flashlight, players, toys, etc.However, in its original "wet" form elements Leklanshe were neither compact nor reliable. Therefore, many innovators have tried many times to improve its consumer qualities, such as placing in airtight containers, not allowing leakage.
Types of batteries
 |
Most modern batteries: nickel cadmium, nickel metal hydride and lithium all - were developed as early as the 20th century in the laboratories of large companies or universities. New chemical systems are not invented by individual fans, based on their own intuition. Basic principles which underpin the functioning of cells, already thoroughly studied and described the exact formulas. Today the main problem facing developers is the selection of optimal components. Chemists distinguish between galvanic cells of two kinds: the first and second. The difference between them lies in how to produce the energy they produce. Elements of the first kind - is disposable batteries, which produce electricity through chemical reactions that result in anode, cathode and electrolyte undergo irreversible changes. This makes recharging of such batteries is impossible or very irrational (for example, to charge certain types of batteries have to spend ten times more energy than they save, while other species may accumulate only a small fraction of its original charge.) After that, the battery will only have to throw in the dustbin, where, hopefully, it gets into recycling and most likely to landfill. Elements of the second kind is more often called batteries. This means that they can be charged if the electrodes to connect the DC source. Chemical reactions occurring in them, are reversible. Thus, the batteries of the second kind do not produce, but only save energy. Other things being equal, the batteries seem to be the best choice compared to disposable batteries. Using them, we do not do as much harm to the environment, because after the discharge, they should not be thrown away. One battery can be used for about a year, while conventional batteries for the same period would need 100-200 pieces, and each element contains toxic substances. But it is not so simple. In fact, the batteries have some serious shortcomings, which do not allow them to displace all other batteries. In urgent cases, disposable batteries are the best choice. They are cheap and always ready to go. But for mobile devices that are used regularly, batteries remain the most profitable option. None of the battery can not store energy forever. Chemicals inside react with each other and gradually decompose. As a result, reduces battery life. In this progressive relaxation are two main reasons. Some chemical reactions affect the ability to store energy. After some time the battery will lose its entire charge. This time, called the shelf life is usually indicated on her body. It depends on the type and design of the batteries, but the storage conditions also affect the duration of their life. Modern lithium batteries can be stored for more than ten years, at the same time, elements of other types can be discharged in a couple of weeks (for example, zinc-air battery after starting use). But even the "long-running" samples can be rendered unusable sooner if they are stored in adverse conditions. Particularly affects the impact of higher temperatures. If they are, by contrast, cool (and some types even freeze), it often helps to keep them in the best shape at a time much longer than the specified expiration date. Reversible chemical reaction in batteries occur even when not in use. This process is called self-discharge. It is reversible, as well as the usual discharge. The rate of self-discharge effect, the same factors as in the period of storage, so it can also be very different for different types of batteries: some lose up to 10% charge per day, while others only 1%. Another indicator that is important to know for each type of battery, it is the specific capacitance.It is defined as the ratio of the element to its mass or volume and is expressed in watt-hours per unit mass or volume. The higher the ratio, the more energy can be stored per unit weight, and the more attractive it is for use in portable devices. This table shows the ratios for different types of batteries, expressed Vatt-chasah/kg.
| Type | Voltage | Ud.emkost |
| Ni-Cad | 1,2 | 40 - 60 |
| NiMH | 1,2 | 60 - 80 |
| Li-Ion | 3,6 | 90 - 110 |
| Li-Polymer | 3,6 | 130 - 150 |
Chemical Systems
One of the most important factors in the development of batteries (as well as any device that feeds them) is to achieve maximum specific capacitance for a given (minimum) size and weight. Chemical reactions occurring inside the element and determine its capacity and physical size. In principle, the whole history of the development of batteries is reduced to finding new chemical systems and package them in a body as small as possible. Today is a lot of different types of batteries, some of which were developed in the 19th century, while others barely noted a decade. Such diversity is explained by the fact that each technology has its strengths. We will tell you about the most common of those used in mobile devices.
Dry cell batteries
The first series-produced batteries were just dry. Heirs of the invention Leklanshe, they are most prevalent in the world. Only one company Energizer sells more than 6 billion such batteries annually. In general, "says the battery, we mean - dry cell." And this despite the fact that they have the lowest specific capacity of all the "massive" types. Such popularity is due, firstly, their cheapness, and secondly, that this name is called at once three different chemical systems: chlor-zinc, alkaline zinc-manganese batteries (elements Leklanshe). Their names give an indication of chemical systems, based on which they were created. In the dry cell batteries located on the axis of carbon rod cathode current collectors. Cathode itself is a system that includes manganese dioxide, carbon electrode and electrolyte. Zinc "cup" is the anode and forms a metal housing element. Electrolyte, in turn, is a mixture, which consists of ammonium chloride, manganese dioxide and zinc chloride. Manganese-zinc and chlor-zinc elements differ, in fact, the electrolyte. The former contain a mixture of ammonium chloride and zinc chloride, diluted with water. In the chlor-zinc electrolyte by nearly 100% is zinc chloride. The difference in the nominal voltage at which the minimum: 1,55 V and 1.6 V respectively. Despite the fact that the chlor-zinc has a greater capacity as compared with the elements Leklanshe, this advantage disappears at low load. Therefore, they often write "heavy-duty", ie elements with high power. Be that as it may, the effectiveness of all dry cell decreases strongly with increasing load. That is why in modern cameras to put them is not worth it, they just are not designed for this purpose. No matter how much running around pink bunnies in advertising, alkaline batteries - it's all the same carbon-zinc minerals came from the 19th century. The only difference is a specially selected blend of electrolyte, which allows to increase the capacity and shelf life of such batteries. What's the secret? This mixture is slightly more alkaline than the other two types.
 |
If the chemical composition of alkaline batteries is little different from the item Leklanshe thereof, the design differences are significant. We can say that alkaline battery dry cell is turned inside out. The outer casing of them is the anode, it's just a protective shell. Anode is a jelly-like mixture of zinc powder mixed with an electrolyte (which, in turn, is an aqueous solution of potassium hydroxide). Cathode, a mixture of coal and manganese dioxide, surrounds the anode and electrolyte.It separates a layer of nonwoven fabric such as polyester. Depending on the application, alkaline batteries can last 4-5 times longer than conventional carbon-zinc. This difference is especially noticeable when using this mode, when short periods of high stress interspersed with long periods of inactivity. It is important to remember that alkaline batteries are not rechargeable, because the chemical processes in which they are based, are not reversible. If it is put into the charger, it will not behave like a battery, but rather as a resistor - will start to heat up. If it does not pull out in time, then it warms up enough force to explode.
Nickel-cadmium batteries
Title tells us that the batteries of this type have a nickel anode and a cadmium cathode. Nickel-cadmium batteries (designated Ni-Cad) are popular among consumers around the world. Last but not least is due to the fact that they can withstand a large number of charge-discharge cycles - 500 or even 1000 - without a significant degradation in performance. In addition, they are relatively lightweight and energy-intensive (although the specific capacitance of approximately two times lower than that of alkaline batteries). On the other hand, they contain toxic cadmium, so that they should be more careful, both during use and when to dispose of.
 |
The output voltage of most batteries decreases as the discharge, because the result of chemical reactions increase their internal resistance. Nickel-cadmium batteries are characterized by very low internal resistance, and therefore may apply to the output current is strong enough that, in addition, virtually unchanged as detente. Accordingly, the output voltage also remains virtually unchanged as long as the charge is almost never run out. Then the output voltage drops to near zero. Constant output voltage is an advantage when designing electrical circuits, but it also makes the definition of the current charge level is practically impossible. Because of this particular balance of power is calculated on the basis of working time and the known capacity of a particular type of battery, and hence is the approximate value. Far more serious shortcoming is the "memory effect". If a battery is not fully discharge and then put the charge, their capacity may be reduced. The fact that such a "wrong" charge at the anode of crystals of cadmium. They play the role of chemical "memory" batteries, remembering that the intermediate level. When, during the next discharge of the battery will fall to this level, the output voltage will drop as if the battery is completely discharged. Vindictive crystals will continue to be formed at the anode, enhancing the effect of this unpleasant effect. To get rid of it, you need to continue easing after reaching this intermediate level. The only way to "erase" the memory and restore full battery capacity. Far more serious shortcoming is the "memory effect". If a battery is not fully discharge and then put the charge, their capacity may be reduced. The fact that such a "wrong" charge at the anode of crystals of cadmium. They play the role of chemical "memory" batteries, remembering that the intermediate level. When, during the next discharge of the battery will fall to this level, the output voltage will drop as if the battery is completely discharged. Vindictive crystals will continue to be formed at the anode, enhancing the effect of this unpleasant effect. To get rid of it, you need to continue easing after reaching this intermediate level. The only way to "erase" the memory and restore full battery capacity. This technique is commonly called a deep discharge. But deep does not mean complete, up to scratch. " This is just hurt and will shorten the life of the item. If in the process of using the output voltage falls below the 1 V (at nominal voltage 1,2 V), it has lead to faulty batteries.Sophisticated equipment, such as PDAs or laptops are configured so that they are turned off before the battery level falls below the threshold. For deep discharge batteries need to use special devices that produce many well-known companies. Some manufacturing companies stated that the new nickel-cadmium batteries are not susceptible to memory effect. However, in practice it has not been proven. No matter what the producers have promised to achieve maximum impact every time the batteries should be fully charged and then wait for the normal discharge that they are not spoiled and have served the entire term.
Preventing electrolysis
As a result of electrolysis in nickel-cadmium batteries can accumulate potentially explosive gases: hydrogen and oxygen. To prevent this, the batteries are placed in a sealed envelope. There are special microvalves designed for automatic venting of accumulated gases. They are so small that they are very difficult to notice. It is important that these valves were closed, so battery life should not wrap, glue or tape winding.
Nickel-metal hydride batteries
 |
From the viewpoint of chemistry, an ideal material for the cathode would be hydrogen. But under normal conditions of use for this is impossible. At room temperature and atmospheric pressure, it is a gas, and it's easier to use for filling balloons, rather than as material for batteries. However, in the late 60th century, the 20 scientists have discovered a number of alloys capable of binding the atomic hydrogen in a volume 1000 times greater than their own. They are called hydrides, a chemical compound, they are typically metals such as zinc, lithium and nickel. With proper use, with the help of the hydrides can store enough hydrogen to be used in reversible reactions inside a battery. The most widely used Nickel-Metal Hydride (NiMH) batteries, which have hydride cathode and a nickel anode. The use of hydrides has several advantages. The most obvious is that the production does not use toxic cadmium. The absence of this material also means that these batteries should be free from memory effect. In addition, through the use of hydrogen as a cathode, managed to finish 50-percent increase in specific capacity (compared to nickel-cadmium batteries). In practice, this means that the nickel-metal hydride batteries, the player or similar device will run on 50% longer. But the use of hydrogen brings not only positive but also negative results. The main drawback is that these batteries are significantly more prone to self-discharge. Some of them are losing up to 5% charge per day, although the latest models, this performance was reduced. Schedule of discharge of nickel-metal hydride batteries under load is slightly different from the nickel-cadmium. The rated voltage, they do not differ (all the same 1.2 V). But if the battery is fully charged, then for some time the output voltage of 1.4 V. After this short period, it drops to 1.2 V, and further NiMH-battery behave the same way as the NiCad. Both types generally have enough similar characteristics. NiMh-batteries can also produce the current of great strength, can withstand a lot of charge / discharge cycles (usually about 500), yet they are two different technologies. If, during battery discharge of the two types behave almost identically, then the charge of similarity is not observed. More specifically, the nickel-cadmium batteries while charging almost do not change its temperature. Nickel-Metal Hydride thus generate heat, and when reaching full charge, they can heat up quite significantly. Because of this, for different batteries require different chargers. And although the market there are universal devices, usually once they can charge the batteries of the same type.
Lithium-ion batteries
Lithium is the most reactive metals, and used it in the most compact systems that provide energy-the-art wireless technology.Lithium cathodes used in almost all batteries with large capacity. But thanks to the activity of this metal batteries are obtained not only very receptive, they also have the highest rated voltage. Depending on the anode, the lithium-containing elements have an output voltage from 1,5 V to 3,6 V!
 |
The main problem in using lithium again, is its high activity. He may even break out - to say nothing, not the most pleasant feature, when it comes to batteries. Because of these problems, the elements on the basis of metallic lithium, which began to appear as early as the 70th-80th years of the 20th century, the "famous" for its low reliability. To get rid of these difficulties, manufacturers have sought to use lithium batteries in the form of ions. Thus, they managed to get all the useful electrochemical qualities, without contacting the whimsical metal form. In the lithium-ion cell, lithium ions are related molecules of other materials. Typical Li-Ion-battery has a carbon anode and cathode of litiykobaltdioksida. The electrolyte is basically a solution of lithium salts.
 |
Lithium batteries have a higher density than nickel-metal hydride. Say, in notebooks such batteries may work in one and a half times longer than nickel-metal hydride. In addition, lithium-ion cells spared from the effects of memory, which suffered early nickel-cadmium batteries. On the other hand, the internal resistance of a modern lithium cells is higher than that of nickel-cadmium. Accordingly, they can not provide such strong currents. If Ni-Cd cells are able to melt the coin, lithium is not able to. But still, the power of these batteries would be enough for a laptop, if it is not associated with jump loads (this means that some devices such as hard drive or CD-ROM, should not cause the high jump at extreme conditions - for example, during the initial promotion or wakes up from sleep mode). Moreover, even despite the fact that lithium-ion batteries will stand for hundreds of charge cycles, they live shorter lives than those that use a nickel. Due to the fact that the lithium-ion cells using a liquid electrolyte (even if separated by a layer of tissue) in the form they are almost always the cylinder. Although this form is not worse than other forms of elements, with the advent of polymerized electrolyte lithium-ion batteries are becoming smaller.
Lithium-polymer batteries
The most advanced technology used today to create a battery that is a lithium-polymer. Already among the manufacturers as batteries, and computer devices has been a tendency for a gradual transition to this type of elements. The main advantage of lithium polymer batteries is the absence of liquid electrolyte. No, it does not mean that scientists have found a way to dispense entirely with electrolyte. The anode is separated from the cathode of a polymer barrier, composite materials such as polyacrylonitrile, which contains a lithium salt. Due to the absence of liquid components, lithium-polymer elements can take almost any shape, unlike other types of cylindrical batteries. Conventional forms of packaging for them are flat plates or bars. As such, they better fill the space the battery compartment. As a result, at the same specific density, lithium-polymer batteries optimum shape can be stored on 22% more energy than comparable lithium-ion. This is achieved by filling the "dead" volumes in the corners of the compartment, which would have remained unused in the case of cylindrical batteries. Besides these obvious advantages, lithium-polymer elements are environmentally friendly and easier due to the absence of an external metal casing.
Lithium-battery zhelezodisulfidnye
Unlike other lithium-containing batteries, which have an output voltage higher than 3V, the lithium-zhelezodisulfidnyh it in half. In addition, they can not be recharged. This technology represents a compromise to which the developers have gone to ensure compatibility of lithium power sources with the technique, developed for use alkaline batteries. The chemical composition of the batteries was specially modified. They lithium anode separated from cathode zhelezodisulfidnogo layer of electrolyte. This sandwich is packed in a sealed enclosure with mikroklapanami for ventilation, as well as nickel-cadmium batteries. This type of element has been conceived as a competitor to alkaline batteries. Compared with lithium-zhelezodisulfidnye weigh a third less, have higher capacity, and, moreover, is also stored for longer. Even after ten years of storage, they retain almost all their charge. Superiority over its competitors are most pronounced at high load. In the case of high load currents lithium zhelezodisulfidnye elements can operate in 2,5 times longer than alkaline batteries of the same size. If the output does not require a high current, this difference is much less noticeable. For example, one of the producers of batteries, said the following characteristics of two types of their battery size AA: at a load of 20 mA alkaline battery will work for 122 hours vs. 135 hours in a lithium-zhelezodisulfidnoy. If the load increased up to 1A, the duration of the work amounted to 0,8 and 2,1 hours respectively. As they say, the result is obvious. These powerful batteries does not make sense to put a device that consume relatively little energy for a long time. They were specifically designed for use in cameras, powerful flashlights, and an alarm clock or radio is better to put alkaline batteries.
Chargers
 |
Modern devices for charging - a sophisticated electronic devices equipped with different systems of protection - both yours and your batteries. In most cases, each type of element to its own charger. When used improperly, can ruin not only batteries but also the charger. There are two modes of chargers - with constant voltage and constant current. Devices that work only with constant voltage is very simple. They have always served the same voltage, but the current strength depends on the level of charge the batteries (and other factors). With the accumulation of energy, the voltage increases and, hence, decreases the potential difference charger and battery. As a result, the current in the circuit decreases. They are arranged is not difficult, all you need - a transformer (to reduce the voltage to the desired level) and rectifier (for straightening the AC to DC). Such devices are equipped with some of the lithium-ion batteries, although they are usually added to the system of protection against over-charging and so on. The second type of charger provides a constant current strength and alters the voltage to provide the required current value. Charging stops when the battery voltage reaches a full charge. Typically, these devices are used for nickel-cadmium and nickel metal hydride elements. In order not to spoil the battery, you need time to stop charging after reaching the desired level. Depending on the type of battery and "tricked" the charger to determine the required time to recharge using multiple technologies. In the simplest cases is measured by the voltage produced by battery. The system monitors the battery voltage and terminates the chain at a time when it reaches a threshold level. But this method is not suitable for all elements. For example, it will never meet in chargers for nickel-cadmium batteries, which discharge curve is almost straight most of the time. This makes it impossible to determine the threshold voltage. More sophisticated chargers selected mode of operation, based on measuring the temperature of the element. When the battery starts to heat up, they will reduce the amperage.Usually in such batteries are built thermometers, which monitors the temperature of the element and transmit a corresponding signal charger. The most advanced devices use these two methods at once. They start with a large current, and then processing the data from the sensor voltage and temperature, can switch to low. If the battery is charged, then they go into maintenance mode charge. In this case, the battery is recharged only slightly to compensate for the process of self-discharge. Charge current in this case is only one-twentieth, one-thirtieth of the nominal discharge current batteries. But for this battery should maintain a small current charging mode (for example, nickel-cadmium batteries charged so you can not). Most chargers for laptops and cell phones are designed in such a way that can be constantly connected to the elements.
Storage
If you want your battery served as long as possible, something about them to care for. With elements of the first kind, ie with disposable batteries, more simply, their only important thing is to store, and after using them all the same throw. Batteries, the elements of the second kind, require more attention, because they need to be regularly recharged. All batteries overheating spoil. And may even become destructive charge, if it does not stop in time. Nothing serious is that your battery to feel warm when it is connected to a charger. But for Overcharging temperature rises significantly, the battery becomes hot, and it is a sure sign that more of its charge will not succeed. Battery can also be rendered unusable if it is completely discharged. This may be caused by short circuit. Incidentally, an interesting fact: some of the battery after the discharge below the recommended level can change the polarity! In general, if your laptop is to alert you that its battery is almost fully discharged, do not try to continue the work - more will come. Most rechargeable batteries are best stored in a discharged state. This particularly applies to Ni-Cd cells. Therefore, those batteries that are long in stock, usually sold uncharged.
Devices
Most of the devices involves the use of a battery of standard sizes, for example, AA, AAA, and the like. Therefore, buyers have a choice, what type of items to choose. The inscription "Heavy-duty" (high load), which can be seen on some carbon-zinc batteries is not just a publicity stunt. This means that they are intended for use in applications requiring a current of great strength. Examples of such devices - lights, electric motors and all the appliances in which they are applied, such as children's toys. There, these batteries last a lot longer than usual. If the device consumes little power, then this advantage is almost is almost unnoticeable. Different lithium-containing batteries are very different from each other in regard to the application. Li-zhelezodisulfidnye are champions at work c heavy loads. Other types, such as lithium watch batteries, used in applications where the load on the contrary, are not large. Lithium-ion and lithium polymer are somewhere in between, and therefore are the most versatile. Where can be used batteries and disposable batteries are usually preferable to first. But in some cases, their benefits are not claimed. Take, for example, remote control, which consumes very little power, but is used continuously and for a long time. Conventional batteries can last it for several years and the batteries do not live so much, in addition to such long periods of time makes itself felt far higher self-discharge rate of these elements. At the other extreme are devices that are rarely used, but must always be ready to work when needed. They are also better to put something disposable, but the "long-playing". In general, the principle is clear - do not have the best battery or battery for each application will be something good and something bad. Well, finally, we repeat some important rules:
- If a metal object short circuit the battery terminals, then it starts to heat up. This can cause damage to your property, and even fire.
- Most of the batteries produces hydrogen in the process of electrolysis caused by recharging. Sealing of buildings of modern batteries significantly reduces the risk of leakage and fire of gas, but no complete assurance can not give because the built-in valves periodically release the surplus accumulated hydrogen.
- Much more dangerous is a gas which can not leave the building. If for some reason the automatic valves were blocked when the temperature inside the pressure may grow so that the battery will explode. Therefore, building batteries should never be sealed, sealed in plastic and the like.
- Almost all batteries contain hazardous chemicals: toxic, poisonous, flammable - depends on technology. It is therefore important that they be properly disposed of after use. Naturally, it is still all that is on the nearest landfill, but it is better to let them lie somewhere far away, than lying on the street.
Sources, Materials:







